推荐试卷
Copyright ©2020 版权所有 广东启点学教育科技有限公司 粤ICP备20024846号
 粤公网安备 44130202000953号
粤公网安备 44130202000953号
 粤公网安备 44130202000953号
粤公网安备 44130202000953号
技术支持:广东启点学教育科技有限公司 4.2.1
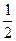 O2(g)=H2O(1) △H=-285.8kJ·mol-1
O2(g)=H2O(1) △H=-285.8kJ·mol-1